INVENTIONS: MATERIALS


Rubber from the field
Diving suits, car tyres, rubber seals – more than 40 000 everyday products contain latex. It is traditionally harvested from the sap of the rubber tree. But as the trees only grow in a tropical climate, German chemist Fritz Hofmann developed a kind of synthetic latex during the First Word War. Today latex – and therefore rubber – is primarily manufactured from mineral oil products.
But other sources have potential as well: for example the Russian dandelion, whose sap contains latex too. Fraunhofer researchers are currently working with Continental, the tyre manufacturer, to build a pilot processing plant that can extract large amounts of dandelion rubber.
» Watch the clip "Natural Rubber from Dandelions"

Russian dandelion (Taraxacum koksaghyz) is a natural source of latex

Car tyres could soon be the first industrial product made from dandelions
Tailor-made steel
One type of steel is not the same as another. A turbine has to withstand different forces in comparison with a car body or a bridge pier. Specialist steels are required so that the structural elements are up to the job. There are already more than 2500 different ones.
The main constituent is always iron. The steel is given its special properties by the addition of other elements – manganese, nickel or chrome: for example it becomes lighter, more stable or more ductile. Scientists can simulate and optimise the properties before manufacturing using computer programs. In this way they can make turbines last longer, aircraft weigh less and cars safer.
Until recently even experts believed that there was hardly anything still to develop with steel. But the innovative steels developed by the German materials researchers prove the opposite.
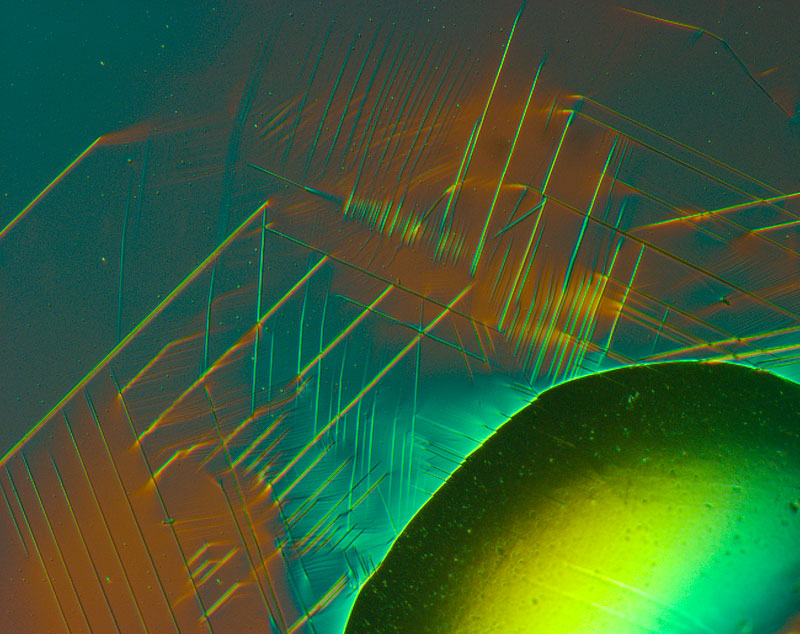
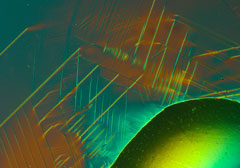
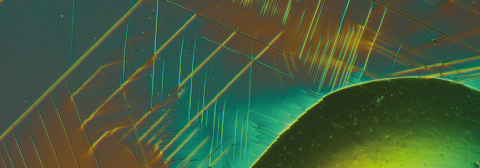
Alloys with various different additives improve the properties of steel

Today steel is optimised for every application, for example aircraft turbines

Carbon in mass production
Helmets, bicycles, tennis rackets, but also aircraft and F1 racing cars, need to be as light as possible, but still provide stability. Carbon fibre reinforced plastics, CFRP for short, make this possible. The disadvantage: until now it has been labour-intensive, which puts up the product prices.
German material researchers are working with specialists in the automotive industry to develop new production processes that will make CFRPs much cheaper. Machines can braid and shape the fibres, and then sheathe them in resin. The finished parts are only half the weight of steel, they are crash-proof and do not rust. An ideal material for economical planes and vehicles – the thing is, the fuel consumption drops along with the weight.

New production processes make carbon fibre reinforced plastics cheaper

Lotus effect
Lotus leaves are always clean – that’s why the plant is considered a symbol of absolute purity in many religions. The myth has a scientific background: water cannot soak into the leaves – it forms beads that take the dirt away with them. German botanist Wilhelm Barthlott found out why in the 1970s: the leaf surface is not smooth, it is covered with microstructures.
Nowadays researchers use the lotus effect to develop specialist surfaces that repel water, oil and even blood. The scientists’ goal: self-cleaning solar cells, dirt-repellent window panes and particularly effective heart-and-lung machines.

Water does not soak into the leaf of the lotus flower

Nanocoated paints use the lotus effect: water forms beads, that roll off, taking the dirt with them

Smart clothing
A firefighter’s jacket with integrated electronics – developed in a government research project – can do far more than just resist extreme heat. In an emergency situation it reliably reports the firefighter’s location, heart rate and body temperature. And the intelligent jacket will notify incident command if necessary, so that they can send in help.
But smart clothing doesn’t just help in an emergency: an intelligent sports shirt that measures breathing and pulse is being developed by the Fraunhofer researchers. This allows sportsmen to optimise their training.

“Intelligent” clothing can save lives

Ubiquitous plastic
Toddlers’ ride-on toys, mop buckets, tubing, bin bags, medical implants – from hi-tech products to everyday objects: polyethylene is one of the most commonly used plastics. It is exceptionally sturdy, does not corrode even when exposed to aggressive substances, and can cope with extreme temperature fluctuation.
Max Planck researcher Karl Ziegler discovered how to produce polyethylene cheaply and quickly in 1953: ethylene gas is converted to polyethylene at room temperature and normal air pressure if certain metal compounds – Ziegler-Natta catalysts – are added. It was only after this discovery that polyethylene could be mass-produced.
However the stability of polyethylene also has disadvantages: plastic bags contribute towards the growing mountains of waste and pollute the environment. It can take several hundred years until a carrier bag breaks down completely, for instance.

Nobel Prize for Chemistry 1963: King Gustav VI Adolf of Sweden congratulates Karl Ziegler (right)